REFERENCES
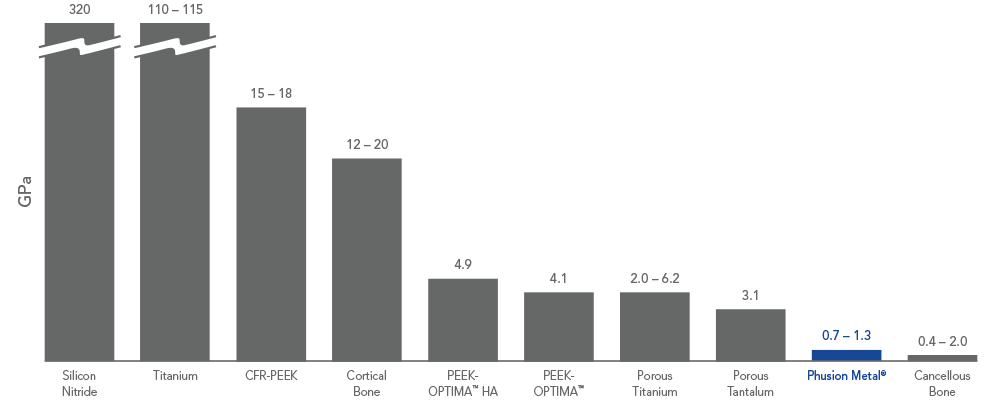
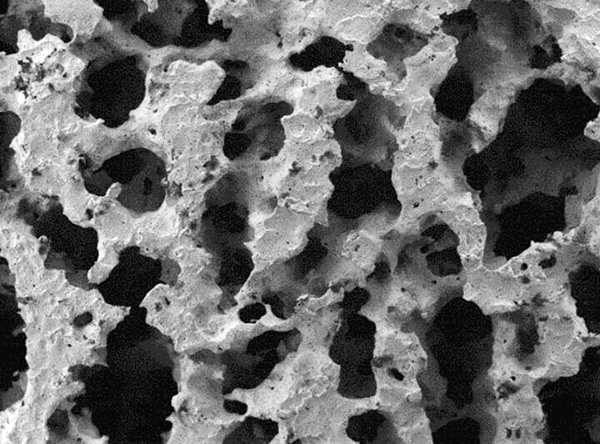
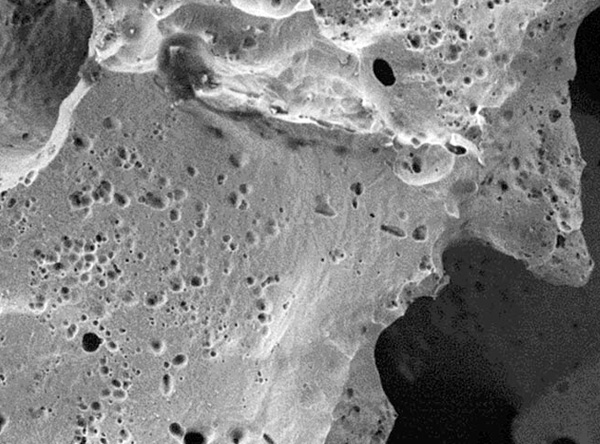
1. Data on file at PorOsteon.
2. Bryan JM, Sumner DR, Hurwitz DE, Tompkins GS, Andriacchi TP, et al. (1996 Sep). Altered load history affects periprosthetic bone loss following cementless total hip arthroplasty. J Orthop Res, 14(5), 762 – 768.
3. Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. (2010 Aug). Bone remodeling around porous metal cementless acetabular components. J Arthroplasty, 25(5), 741 – 7.
4. Bobyn JD, Mortimer ES, Glassman AH, Engh CA, Miller JE, et al. (1992 Jan). Producing and avoiding stress shielding Laboratory and clinical observations of noncemented total hip arthroplasty. Clin Orthop Relat Res, (274), 79 – 96.
5. Chen Y, Wang X, Lu X, Yang L, Yang H, et al. (2013 Jul). Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J., 22(7), 1539 – 1546.
6. Niu CC, Liao JC, Chen WJ, Chen LH. (2010 Jul). Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech, 23(5), 310 – 6.
7. Zhu Y., Yang R. et al. (2009 May). Effect of Elastic Modulus on Biomechanical Properties of Lumbar Interbody Fusion Cage, J Master Sci Technol, 25:3, 325 – 328.
8. Kumar N, Judith MR, Kumar A, Mishra V, Robert MC. (2005 Aug) Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976), 1;30(15), 1731 – 1735.
9. Shirazi-Adl A, Dammak M, Paiement G. (1993 Feb). Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J Biomed Mater Res, 27(2), 167 – 175.
10. Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. (2014 May). Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg, 6(2), 81 – 89.